The continuity of memory is like a forward-flowing river of time, carrying the journey of eternal moments.
Spanish surrealist Salvador Dalí illustrated this concept in his famous work "The Persistence of Memory": soft clocks hanging eternally from the branches of life. However, in the era of quantum mechanics, this ever-contemplative artist deconstructed his creation in a work titled "The Chromosome of a Highly Coloured Fish's Eye Starting the Harmonious Disintegration of the Persistence of Memory," later known as "The Disintegration of the Persistence of Memory."


▷ Top: "The Persistence of Memory"; Bottom: Initially named "The Chromosome of a Highly Coloured Fish's Eye Starting the Harmonious Disintegration of the Persistence of Memory," later known as "The Disintegration of the Persistence of Memory." Source: Wikipedia.
Yet, Dalí might not have anticipated that the fragmentation of DNA contained in chromosomes could be a significant source of new memories. Recently, Vladimir Jovasevic and his colleagues at Northwestern University's Feinberg School of Medicine provided an important explanation in an article in Nature: the damage and repair of double-stranded DNA in certain discrete neurons can initiate innate immunity, becoming a source of memory.

▷ Vladimir Jovasevic, Vladimir, et al. "Formation of memory assemblies through the DNA-sensing TLR9 pathway." Nature 628.8006 (2024): 145-153.
1. Starting from Semon: Memory Exists in Specific Clusters of Cells
Does the brain store memories? This seemingly obvious question in contemporary neuroscience was hotly debated among leading scholars in the field of learning and memory a century ago. For some, it was clear that memory was physically represented in the brain, while others believed it was stored in a more spiritualized mind.
In 1904, Richard Semon wrote two monumental works (Die Mneme and Die Mnemischen Empfindungen) proposing a physical theory of human memory.
He introduced the term "engram," suggesting that the brain's state and structure respond physically to a person's experiences. This response implies the existence of a "neural substrate" in the brain for storing and reproducing memories. Once formed, the engram enters a dormant state and can be reactivated when past events recur.
The development of experimental science gradually confirmed the physical existence of this concept.
Karl Spencer Lashley, a pioneer of physiological psychology, initiated the search for the "engram." By lesioning different parts of the cerebral cortex and observing the behavior of rats in mazes, he ultimately failed to find the engram. Yet, despite his frustration, Lashley remained convinced of the engram's existence—he believed it was distributed: "It's not about finding where the trace is, but rather where it isn't."[1]
A significant leap in engram research occurred when Lashley's student, psychologist, and memory theorist Donald O. Hebb proposed the cell assembly theory, similar to Semon's engram complex. Hebb hypothesized that a cell assembly forms among interconnected cells that are simultaneously active during stimulation. Sufficient activity within the cell assembly induces growth and metabolic changes, strengthening the connections between these cells. These synaptic and metabolic changes (potentially including alterations in intrinsic neuronal excitability) affect the function of the cell assembly.
With the advent of more advanced technologies, the search for memory cells underwent a profound revival. By using labeling tracers and targeted interventions on individual neurons (activating or ablating them), researchers finally identified a group of neurons: these neurons exhibit a rapid high expression of immediate early genes during memory formation. Ablating these neurons prevents memory formation (necessity), while artificially activating them induces memory formation (sufficiency) [2].
The discovery of engram cells demonstrates that memory indeed resides in specific populations of neurons and their interactive circuits.
Now, neuroscientists are working to determine how neuronal groups in the hippocampus respond to stimuli that induce memory.
2. DNA Damage and Repair in Engram Cells
"Engrams" refer to the enduring offline physical and/or chemical changes induced by learning, which form the basis of newly established memory associations. Neurons with these physical memory traces (memory engram cells) are typically those that express immediate early genes during specific experiences [3].
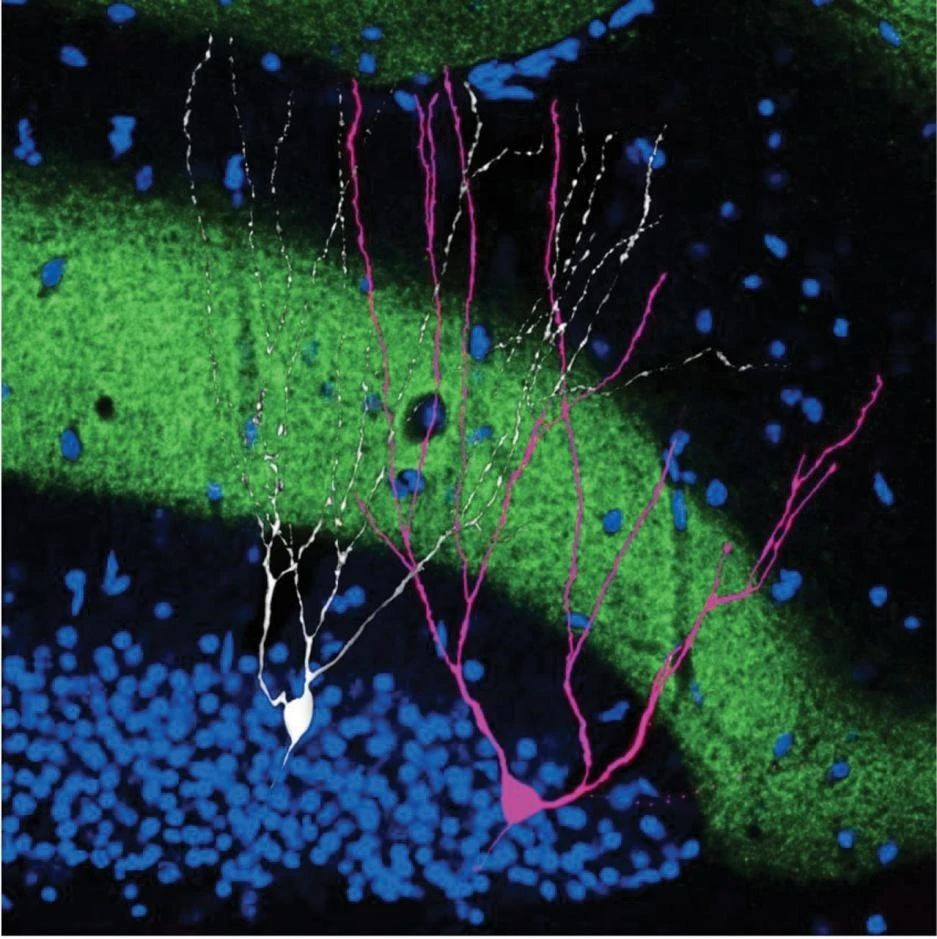 ▷ Engram cells (pink) adjacent to non-engram cells (white). Source: Science
▷ Engram cells (pink) adjacent to non-engram cells (white). Source: Science
It is generally believed that neuronal activity under external stimuli can induce the expression of immediate early genes in engram cells, providing the physical basis for engram formation.
In 2015, a research team led by Professor Li-Huei Tsai from the Department of Brain and Cognitive Sciences at MIT used molecular and next-generation whole-genome sequencing methods to discover that neuronal activity stimulation can trigger the formation of DNA double-strand breaks (DSBs) in the promoters of immediate early genes (IEGs) such as Fos, Npas4, and Egr1. Even in the absence of external stimuli, targeted induction of DSBs in the promoter regions of IEGs is sufficient to induce their expression [4].
The expression of immediate early genes is typically constrained by DNA topology, and DNA double-strand breaks can help rapidly relieve these constraints. Thus, DNA double-strand breaks play a crucial role in the expression of immediate early genes, learning, and memory.
3. The History of DNA Double-Strand Breaks and Memory
Maintaining DNA integrity is crucial for the faithful expression of genes. However, DNA is susceptible to various forms of damage during normal cellular functions. It is estimated that each cell may experience up to tens of thousands of DNA damage events daily, including base mismatches, oxidative base damage, single-strand breaks, and double-strand breaks (DSBs). All types of DNA damage can lead to adverse outcomes such as apoptosis or tumorigenesis. Among them, DSBs are considered the most cytotoxic as they pose a significant threat to the preservation of genetic and epigenetic information and can signal cell death. It is well-known that ionizing radiation-induced DSBs are a major pathogenic factor behind mutations, chromosomal aberrations, genetic instability, and carcinogenesis.
However, not all DNA double-strand breaks are pathological. In healthy functioning cells, DSBs formed during mitosis are repaired through homologous recombination; in damaged or non-dividing cells, DSBs are primarily repaired through non-homologous end joining. These two mechanisms ensure the faithful and efficient repair of DSBs, playing a crucial adaptive role in the immune system and maintaining the delicate balance between damage pathology and adaptive immunity.
In the central nervous system, the balance of DNA damage and repair in relation to memory appears to be more elusive. With aging and disease, DSBs increase. Studies show that DSBs in the hippocampus are considered detrimental to learning and memory throughout the lifespan. Mice repeatedly exposed to low-dose radiation exhibit persistent DSBs in the hippocampal dentate gyrus, leading to reduced neurogenesis and ultimately lower survival rates [5].
At the same time, scientists have long suspected that DSBs might be involved in the memory process. After contextual fear memory retrieval, the active transcription marker histone H3 lysine 4 trimethylation (H3K4me3) increases in the hippocampus, which is one of the signs of DSBs. However, it is unclear how this epigenetic mark is regulated during reconsolidation [6].
If DSBs are involved in learning and memory, then pharmacologically inducing DSBs would seem counterintuitive as it would impair memory. One possible reason is that an increase in DSBs without behavioral experience might be harmful and could inhibit DSBs triggered by behavioral experiences. Another possibility is that drug-induced and behavior-induced DSBs might jointly trigger DSBs in specific regions, whereas individual DSB events might not be sufficient to induce memory formation. Additionally, it is possible that some memory signals are associated with the repair of DSBs.
These data suggest that DSBs have an adaptive role in the central nervous system.
4. A New Cluster of Neurons, Distinct from Engram Cells
So far, DNA double-strand breaks (DSBs) seem closely associated with engram cells, providing a pathway to explore how these cells function.
Jovasevic's team discovered another group of neurons. They found that in a distinct neuronal population different from those expressing immediate early genes, DNA damage repair induced by fear memory became a source of long-term memory. DNA damage induced by memory stimuli could be linked to persistent cellular changes associated with long-term memory.
Jovasevic's team conducted contextual fear conditioning training on mice and found that:
(1) Four days after training the mice for fear conditioning (considered a short-term memory period), DNA and nuclear damage appeared in the hippocampus of the mice.
(2) Observing the mice 21 days after fear memory stimulation (considered a long-term memory period), they found signs of inflammation in the hippocampus, activation of the Toll-like receptor 9 (TLR9) signaling pathway, and triggering of the cytosolic DNA innate immune response. This response is usually the body's defense mechanism against bacterial infection.
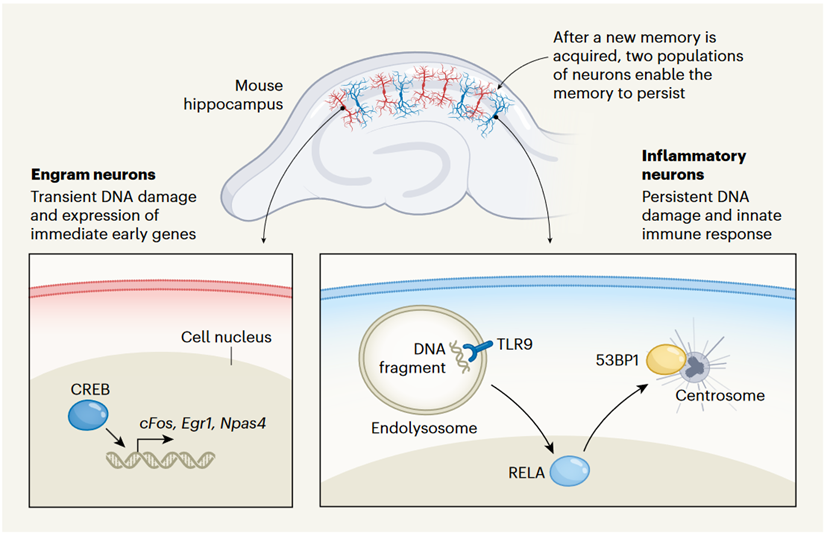 ▷ Mechanism of memory formation in inflammatory neurons and engram neurons. Source: Nature
▷ Mechanism of memory formation in inflammatory neurons and engram neurons. Source: Nature
Although most DNA DSBs are rapidly repaired, those that persist show a specific centrosomal localization. The centrosome, known for its role in cell division, also plays an increasingly important role in the DNA damage response.
Within the centrosome, DNA forms a crucial damage response complex, including the protein 53BP1, a key mediator of DSB repair. Studies show that removing Toll-like receptor 9 from hippocampal neurons prevents the accumulation of centrosomal DNA damage and DNA repair complexes, indicating that TLR9 signaling's critical function in neurons is maintaining genomic integrity.
Jovasevic et al. further demonstrated how the transcription factor RELA localizes to the centrosome. These findings establish a molecular-level link between memory acquisition and its long-term reliable storage, providing substantial insights into the identification of these inflammatory neurons.
So, how do these specific clusters of inflammatory neurons interact with engram cells? Researchers speculate that inflammatory neurons may play a crucial role in the stability and flexibility of memory, while engram neurons are responsible for triggering memory recall.
Studies on engram neurons show that the baseline expression of the transcription factor CREB is the promoter of activity-induced gene expression in memory, initiating the recruitment of individual neurons into the engram. A similar mechanism may exist in the inflammatory cell population. DNA damage could cause some neurons to undergo persistent DNA damage and an innate immune response post-learning.
The authors hypothesize that engram neurons might generate the initial memory signals, while inflammatory neurons support and shape the memory, helping it persist over time.
5. Back to Semon: Memory and Inheritance
In his book Die Mneme, Richard Semon proposed a broader unified theory emphasizing the similarities between memory and inheritance. This stemmed from his simple observation that parents' learning and experiences could be transmitted to their offspring. This idea of "soft inheritance" originally came from Lamarck.
Compared to the engram theory, Semon's hypothesis of intergenerational memory inheritance seemed more sensational. Once proposed, it was cast aside along with Lamarck's ideas, only to be picked up by the most optimistic of revivalists.
In the 1950s and 60s, James V. McConnell, an experimental psychologist at the University of Michigan, and his colleagues used planarians to study memory processes, sparking a revival of the concept of soft inheritance of memory. He experimented with the regenerative capacity of planarians, bisecting a trained planarian and observing that both new worms retained some memory, demonstrated by their quicker completion of training tasks. Even more astonishing was that when trained planarians were ground up and fed to untrained ones, the latter also completed training tasks more readily, as if memory was transferred through material ingestion.
These incredible experimental results sparked widespread controversy. A significant portion of the scientific community was skeptical, believing that similar conclusions could be reached with proper controls and accounting for inevitable observer bias.
Due to prolonged scrutiny, McConnell became a target in the public eye, even receiving a letter bomb that resulted in his hearing loss.
However, in 2013, a team led by biologists Tal Shomrat and Michael Levin from Tufts University in Massachusetts published a paper fundamentally supporting McConnell's findings. They used different floor textures and varying lighting as cues for adaptive memory, testing decapitated worms' rapid adaptation to previous environments [7].
That same year, a team led by Brian Dias from Emory University in the U.S. discovered the possibility of intergenerational inheritance in mammals. They found that mice could pass on an olfactory fear memory of acetophenone to their F1 generation. The idea of intergenerational memory inheritance, once discarded, was brought back into consideration [8].
As previously mentioned, DNA damage gradually accumulates post-translational modifications of histones, which may be crucial for memory formation. Similar to epigenetic research, the repair of DNA double-strand breaks (DSBs) might be a vital component of transmitting information to offspring.
Using chromatin immunoprecipitation (ChIP) analysis, researchers found significant overlap between DNA DSBs and histone H3 lysine 4 methylation (H3K4me3) in the CA1 region of the hippocampus during memory reconsolidation. Additionally, the phosphorylation of histone H2A (H2A.XpS139) and H3K4me3 levels were associated with long-term memory impairment, suggesting that DSB repair plays an indispensable role in the memory reconsolidation process. Moreover, the monoubiquitination of histone H2B at lysine 120 (H2Bub) is crucial for hippocampal memory formation [6].
Overall, increased DSBs enhance epigenetically mediated transcriptional control, potentially representing a new mechanism for memory reconsolidation. The story of the engram seems to have returned once again to Semon.
6. Outlook
Of course, defining a new cluster of cells cannot rely on isolated findings alone. The discovery of engram cells caused a wave in the academic community a decade ago, supported by a robust triad of verification: observational studies, necessity through knockout studies, and sufficiency through knock-in studies.
Regarding Jovasevic's research, determining how to identify these specific inflammatory neurons and distinguish them from other neuronal populations remains a current challenge. Especially considering that DNA damage plays a crucial role in aging and neurodegeneration, the emergence of these inflammatory neurons raises an urgent question: Toll-like receptor 9 (TLR9) activation typically involves microglia, the central nervous system's immune cells, which usually leads to neurodegeneration. So why, in neurons, does TLR9 activation contribute to memory formation instead?
How can we differentiate between harmful DNA damage and inflammation and the immune response essential for memory?
The activity or functional characteristics of this neuronal cluster in the processes of memory acquisition, consolidation, or retrieval remain unclear. Throughout the memory process, recording electrical activity and selectively manipulating inflammatory neurons are necessary to better understand these cells' contribution to memory persistence.
Answering these questions will require significant effort. Just as Dalí depicted memory and its instability in his iconic surrealist style, the neuronal cluster discovered by Jovasevic and colleagues still needs a comprehensive framework to support the persistence and disintegration of memory.